Celebrating 10 years of Chemical Science: No longer completely in the dark

Professor Hemamala Karunadasa has published over seven research papers with Chemical Science, one of which was recently chosen as our ChemSci Pick of the Week. To celebrate our 10th anniversary, we took the opportunity to catch up with her about her exciting research in the area of perovskites.
Hemamala’s research focuses on the preparation of solid-state materials using the tools of solution-state chemistry. Through careful design, Hemamala and her group prepare new materials that can be utilised for clean energy applications.
Where do you look to for inspiration for your research?
Making connections between different areas in chemistry is something that I really enjoy and serves as a source of inspiration. Recently, we’ve been making connections between families of materials that historically are considered to be different, but actually show important overlap in properties. Layered double perovskites and layered transition metal dichalcogenides belong to two very different families but they both show very intriguing band gap changes when the 3D lattice is thinned to a monolayer, something that was demonstrated in one of our recent Chemical Science papers.
I like where we are in our research now because we have some understanding of how to design the materials that we want, with the properties that we desire. We are no longer completely in the dark.
What has been the most exciting moment of your career so far?
When I was a student, I was fooling around in the lab trying to figure out if a molecule that I had synthesised was a good electrocatalyst. I thought it would be cool to use a mercury electrode, but I didn’t have one – so I made it myself. It was awful, a complete hack job! I tried the catalysis and the solution turned white. I thought the experiment had failed but then I looked more carefully and the whole container was full of bubbles. It was forming hydrogen! I thought something must be wrong, so I stayed in the lab all night to do every control I could think of, but then I realised that it was actually the catalyst working and it was such an exciting moment.
What has been the most challenging moment of your career so far?
Nothing that I have faced as a professor has been as challenging as making the initial decision to become a professor. It seems so overwhelming and difficult, and the thought of starting a lab from scratch is incredibly daunting. I found that thinking about it was scarier than actually doing it – taking things a step at a time until the group builds a momentum of its own. I think this is important for young researchers to hear.
The reason that I had a certain amount of self-confidence to try to become a professor was that even as a student, I was independent. I was very lucky to have some wonderful teachers and advisors, who allowed me to stumble and figure things out for myself. So I thought I could do that again, as a professor. Grad school taught me to be comfortable in the dark.
Which Chemical Science publication are you most proud of and why?
I truly am very proud of all of them. We spend so much time on our papers that by the time a paper is finalised, it is our prize. I think that some of our most important papers have been in Chemical Science. I have been really happy with the way our papers have been received and reviewed.
What do you feel has been the most important development with your area of research since your first publication in Chemical Science in 2015?
My 2015 paper was a collaboration with Michael McGehee. I think this remains the coolest phenomenon that I have seen in 3D halide perovskites. Basically, you can make these halide perovskites with a mixture of halides, like bromides and iodides, which are fully mixed in the lattice. When you shine light on it, the bromides and iodides appear to segregate, which alone is not particularly noteworthy because many materials decompose with light. What was really shocking was that in the dark they go back. The reversibility was so strange. This paper made a huge impact in the field, because it was the first evidence that these solar absorbers are ionic conductors. Due to all of the previous success in this field, we already knew these systems were good photoconductors, but it turns out that they are actually mixed electronic-ionic conductors.
Following on from this work, many groups worldwide have studied this phenomenon, and some beautiful work has been published in trying to understand this strange phenomenon. A number of unusual effects in halide perovskites start to make sense when you realise the high degree of halide mobility in these lattices.
One of your articles was chosen as our "Pick of the Week" back in 2019, and an infographic was prepared to describe the work. Did you find this to be a useful process?
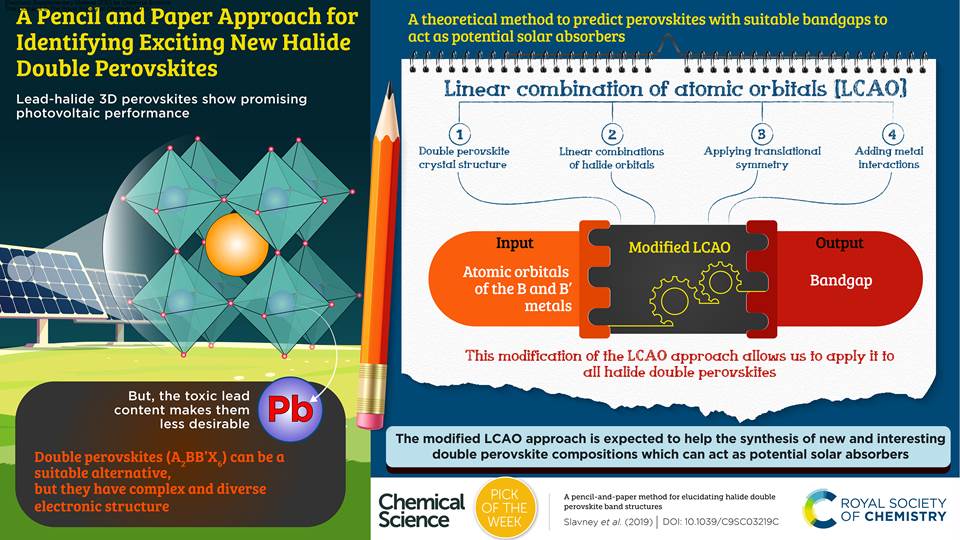
The quality and presentation of the graphic was outstanding. I continue to use it in any way that I can. It is a better figure than we could have made.
It was great to have this paper highlighted. We spent a lot of time trying to make this paper broadly accessible. But we were worried that no one would want to read it, being based on group theory, which almost everyone remembers learning as an undergraduate student but don’t necessarily want to spend more time reading! Through the graphic, the key points were distilled down, which really helped to show readers what you can get out of the paper. I have actually received emails from faculty members saying that they use it in their classes, which makes me very happy. I think it really helped to highlight the basic message. It’s a very powerful technique that helps us really understand our materials.
Overall, I think that Chemical Science is very receptive to fundamental advances in the field. The perovskite field is very application focused. We have sometimes had a hard time publishing our papers that are more fundamental even when it sets the stage for later technological advances. Chemical Science has been very receptive to these papers. Your editors are very knowledgeable in this area.